Nature and relationships of Sahelanthropus tchadensis
Roberto Macchiarelli1 (chercheur) et la classe de Terminale-EURO du Lycée général et technologique Joliot Curie de Hirson de Mme Marion Baudrand2 (la liste des élèves est mentionnée en fin d’article)
Article original/Original article: R. Macchiarelli, A. Bergeret-Medina, D. Marchi, B. Wood, Nature and relationships of Sahelanthropus tchadensis, Journal of Human Evolution, Volume 149, 2020, 102898, doi: 10.1016/j.jhevol.2020.102898.
Institution: 1Unité de Formation Géosciences, Université de Poitiers, 86073, Poitiers, France – Département Homme & Environnement, UMR 7194 CNRS, Museum national d’Histoire naturelle, 75116, Paris, France.
2Lycée général et technologique Joliot Curie, 1 rue de Chanzy, 02500 Hirson
Abstract:
A cranium (a skull without the mandible) of the Sahelanthropus tchadensis species was found in Toros-Menalla, Chad. It was deformed but the discoverers proposed that it belonged to a hominin. In 2001, they found at the same place a part of a very damaged femur, eaten by carnivores. After studies in 2004, the femur seemed to belong to a primate and so it was concluded parsimoniously that it probably belonged to the same species. In this article, this femur is studied to determine if it, and thus the cranium, come from a hominid (that is, if it belonged to a lineage before the shared ancestor with chimpanzees/bonobos) or a hominin (the “human family” after the common ancestor with chimpanzees/bonobos). Morphological and biomechanical features associated with bipedalism were studied to determine if the femur belonged to a habitual biped, and so to a hominin.
This study compared the femur of S. tchadensis to another primitive potential hominin from Kenya (Orrorin tugenensis) and concluded that they had different locomotion modes and didn’t belong to the same species. This study also concluded that S. tchadensis was probably not a habitual biped and so was likely to be a hominid rather than a hominin. Nevertheless, more material from this species (more numerous and better-preserved fossils from other body parts) is needed to do more comprehensive studies to have a more confidence in the results of this study and its hypotheses on this issue.
Key Words: Hominid ; Hominin ; Sahelanthropus tchadensis ; Femur
I. Introduction
There are several scientific studies that indicate that the living taxa (groups) closest to humans are bonobos and chimpanzees because of their morphology, molecules and genetics (Ruvolo, 1997; Prado-Martinez et al., 2013; Diogo et al., 2017). Studies of the genetic clock concluded that hominins (the “human family”) and panins (chimpanzees/bonobos lineage after the common ancestor with Homo) have been separated for about 8-6 million years (Myr) (Bradley, 2008; Stone et al., 2010), but other studies indicate it may have been earlier (Langergraber et al., 2012; see also Moorjani et al., 2016). Two possible extinct hominin species are known from around 8-6 Myr in Africa (so at the supposed time of separation between hominins and panins):
- The first, Orrorin tugenensis, was defined from dental and body remains (except the cranium) recovered from sediments dating to around 6.0 Myr and located in Kenya (Senut et al., 2001), and is commonly considered a bipedal early hominin (Pickford et al., 2002).
- The 2nd species, Sahelanthropus tchadensis, was at first defined from 6 fossils, including the “main” specimen (holotype) which is an adult cranium (Brunet et al., 2002). These fossils were recovered in the same locality in Chad.
Further specimens representing S. tchadensis were recovered in 2001 and 2002, including an upper premolar, and two mandibles (Brunet et al., 2005) and seem to confirm the hypothesis that all these remains belong to only one species. Currently, the remains attributed to S. tchadensis come from six to nine adult individuals from three fossiliferous localities spread over approximately 0.73 km². Studies on absolute dating (Lebatard et al., 2008) assign an age between 6.38 Myr and 7.22 Myr to the sedimentary formations where the remains were found, whereas biochronology (relative) dating studies determined an age between 6-7 Myr (Vignaud et al., 2002). Which means that the fossils would have the same age as the formations, assuming the fossils were found in situ in sediments, but even if the discoverers said that the cranium was found still partly buried, so could be considered “in situ” (Brunet et al., 2004), this has been disputed by other scientists (Beauvilain and Watté, 2009).
The cranium of S. tchadensis is complete, but some areas are deformed, and others are cracked. The preserved elements show that the cranium includes a certain number of primitive characters (i.e., ape-like), but also derived characters (close to more recent hominins) (Guy et al., 2005). These characters are synthesised in the Figure 1.
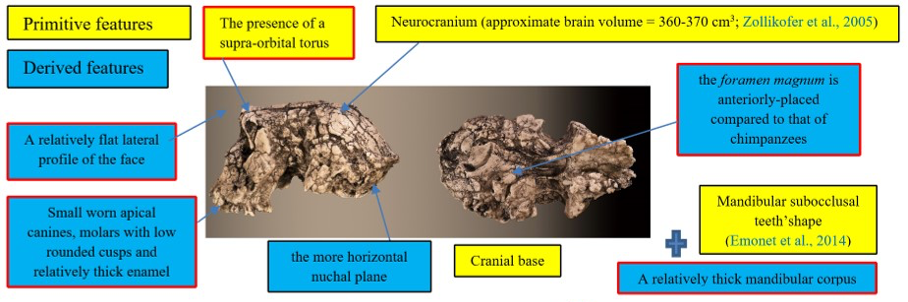
Figure 1: Synthesis of the primitive and derived characters of Sahelanthropus tchadensis (features excluding Sahelanthropus tchadensis from a close relationship with the Pan clade according to Brunet et al., 2002; Guy et al., 2005.)
Some of the characters were used by the discoverers of the fossil to exclude this species from the group of chimpanzees/bonobos (Pan clade, or panins), particularly the advanced position of the foramen magnum, yet some bonobos have such an advanced foramen magnum (Ahern, 2005). Due to the deformities, virtual reconstructions of the cranium have been performed to try to reconstruct its original shape. These reconstructions allowed Zollikofer et al. (2005) to say that the deformations necessary to make the deformed S. tchadensis cranium into a cranium of gorilla or of chimpanzee are too great, which would exclude it from both groups (Wolpoff et al., 2002, 2006). However, using the same principle, the deformations necessary to make this cranium a Homo sapiens are even greater, so this should also exclude it from the hominin lineage. Another study (Guy et al., 2005) based on a virtual reconstruction and a comparison with current and fossil species made it possible to conclude, in a more nuanced way, that the cranium probably belonged to a hominin but that certain characters were either new or combined in a new way in this species and therefore, that some of them could be convergent characters (i.e., not inherited from a common ancestor), which means that some characters are probably not relevant to use to establish relatedness.
However, one characteristic should be highlighted: virtually reconstructed cranium the position of the foramen magnum is closer to H. sapiens than to Australopithecus according to Zollikofer et al., 2005: fig. 4). A phylogenetic study from Mongle et al. (2019), based on updated (from Strait and Grine, 2004) morphological characteristics of the cranium and the teeth concluded that, statistically, there was little chance that this species was a hominin (41%) and that these chances were lower compared to another primitive hominin species, Ardipithecus ramidus (64%). However, it is very difficult to reconstruct relatedness based on few fossils (Smith, 2005) that are deformed, or based on their virtual reconstruction (Brunet et al., 2002, 2004, 2005; Brunet and Jaeger, 2017). The study of fossils of other parts of the body of this species having the potential to provide information on its mode of locomotion (type of bipedalism) are specifically necessary to clarify the relationships between this species and other hominid/hominin species. This is what this article proposes by presenting a study of a femur portion likely belonging to this species (so, the first fossil part of the body and not of the cranium).
The partial femur
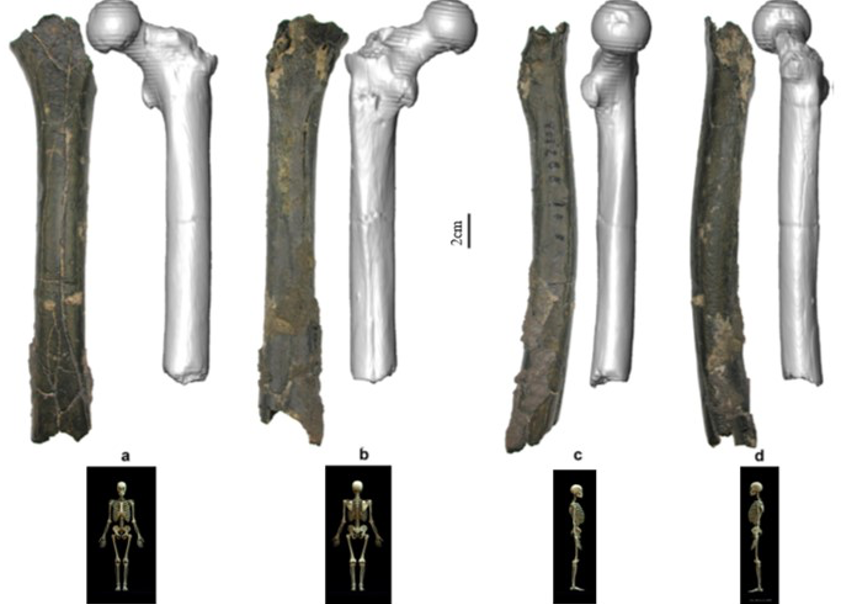
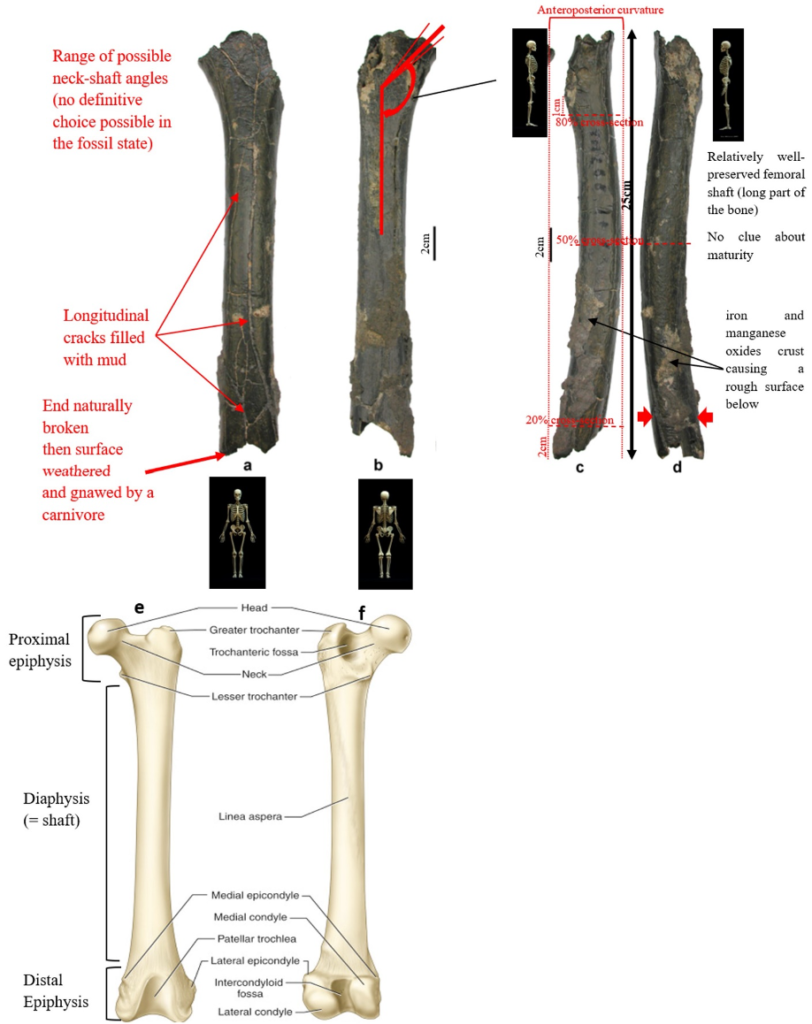
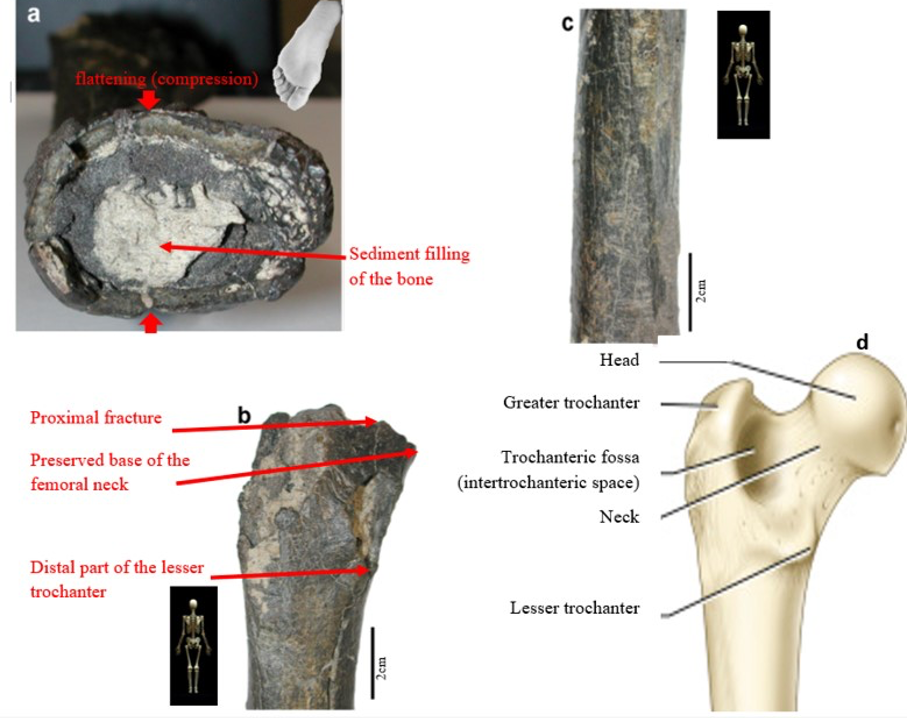
According to Beauvilain and Watté (2009), the partial left femur discussed in this article was collected on 19 July 2001 in the same location as the cranium of S. tchadensis. It was recognized as a probable primate femur (by A.B.-M.) in 2004 during a study on the deposition and fossilization ways of the late Miocene nonhominin fossils of vertebrates from Toros-Menalla (Bergeret, 2004). These fossils, and so the femur, were at the time temporarily stored at the University of Poitiers, but the present whereabouts of the femur are unknown. On Figure 3a, an interval of angles between the femoral neck and the shaft is given because the real angle can’t be known for certain, since part of the neck is missing. However, the space between the trochanters is well preserved (Fig. 3f). On the second picture 3b, some cracks can be observed on the femur because of its depositional history (sedimentary compression). The Figure 3 shows that the distal (lower) part is slightly deformed.
The last Figure 3d also shows that a part of the femur is missing. The surface of the bone became rough because of its oxidation in the sediments. Moreover, part of the femur was flattened (see slightly convergent red arrows) because the femur was buried underground during a long time. It is estimated that the distal break is close to what would have been the junction between the diaphysis and the distal epiphysis (see Fig. 3a & 3e), so a reasonable estimate of the length (Ruff, 2002) of the femur is >280 mm (SOM Fig. S2).
The past fauna from Toros-Menalla included both hyaenids (e.g., Chasmaporthetes, Belbus, Hyaenictitherium, and Werdelinus) and felids (e.g., Dinofelis, Machairodus, Lokotunjailurus, and Tchadailurus; Vignaud et al., 2002; Bonis et al., 2005, 2007, 2010a, b; Peigné et al., 2005; Le Fur et al., 2014), so the possibility that the studied femur belonged to a carnivoran was considered but then rejected as a likely one since in carnivorans the neck-shaft angle is usually lower than the range of possible angles estimated for this femur (see Fig. 3b & 3e). Moreover, in carnivorans the part of the shaft near the pelvis (proximal) is typically not flattened like in this specimen, and the region between the trochanters (see Fig. 3a & 3e) has a characteristically medially-directed crest that should have been apparent in what is preserved of the lesser trochanter, but that is absent. Finally, the fossil femur is curved towards the front throughout its length (Fig. 3c), whereas carnivoran femurs are also somehow bowed, but normally only in the distal part of the shaft (Pale and Lambert, 1971; Werdelin and Lewis, 2001; Werdelin, 2003; France, 2011; see also https://www.archeozoo.org/archeozootheque/). Given that the entire set of other fossils from Toros-Menalla compatible with a large-bodied primate has been assigned to S. tchadensis and that the cranium and the femur were found in the same location (Beauvilain and Watté, 2009: fig. 1a), it is reasonable to assume that the femur should also be assigned to S. tchadensis.
II. Materials and methods
1. Comparative materials
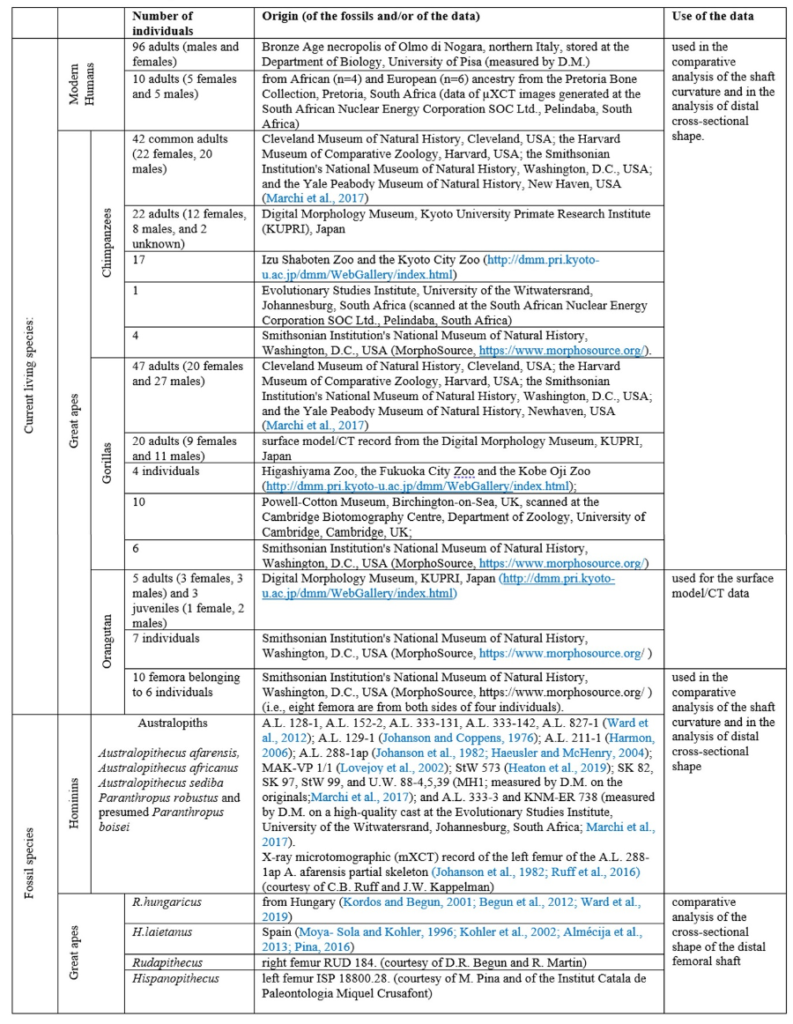
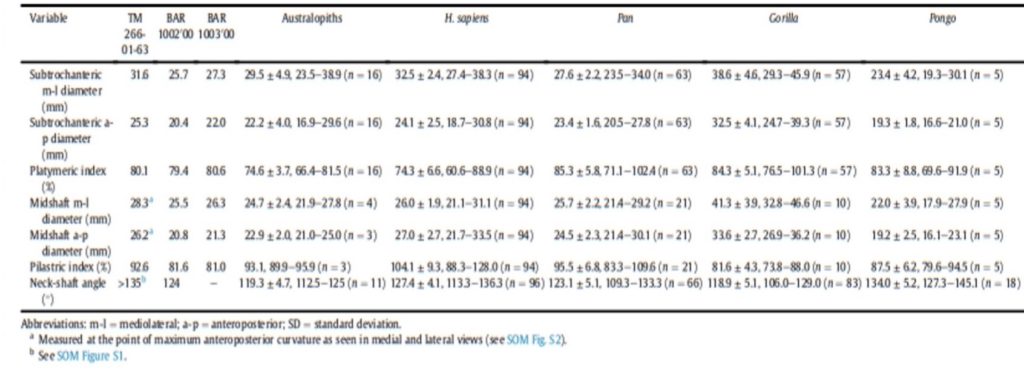
The material used to compare the characteristics the femur with the characteristics of femora of other species to try to characterize its locomotion pattern included current species (Humans and Great Apes) and fossil species (Australopiths and Great Apes). The number and origin of the materials used for the comparison are described in Table1.
2. Methods
Different measurements that could have a significance to characterize the bipedalism were realized on the femur and on femora of the material described in Table 1. The neck-shaft angle (Fig. 3b), the 80%, 50% and 20% cross-sections, and the degree of anteroposterior curvature of the shaft (Fig. 3c) were used to characterize the locomotion of each group (Modern Humans, Chimpanzees, Gorillas, fossil hominins, fossil Great Apes) and compare S. tchadensis with them to try to identify the characteristics of its possible bipedalism.
Measurements were also taken on two femora of O. tugenensis (when possible) to assert its bipedalism and compare it with S. tchadensis.
The characterization then the comparison of the locomotor pattern of each group with the fossils were realized through morphometric analyses (Procrustes analyses, Principal Component Analyses and then between group PCA). These analyses used chosen measurements to characterize a shape and then allow to reveal the principal factors modifying one shape into another. These factors could then be used to try to identify the differences between diverse types of locomotion characterizing each group, and so inferring S. tchadensis’ locomotion.
More specifically, the neck-shaft angle (cf. Köhler et al., 2002) was measured using the software ImageJ (Schneider et al., 2012) on different images of the femur in posterior view. Comparative values of the neck-shaft angle for Pan, Gorilla, and Pongo are a combination of our original (m)XCT-based measurements and data from Pina (2016). The cross sections (15%, 20%, 50%, 80%) of the femur were defined using previous studies (Ruff et al., 1999; Ruff, 2000, 2002; Puymerail et al., 2012; see SOM Fig. S2 and Fig. 3c). For assessing the degree of anteroposterior curvature (Fig. 3c) of the femoral shaft, analyses on the sketch of the femur (SOM Fig. S3) were realised and on the similarly-oriented virtual rendering of O. tugenensis, H. sapiens, Pan, Gorilla, and Pongo femora. The software TpsUtil64 (Rohlf, 2005) digitized the shaft curvature, then generalized Procrustes analyses and a Principal Component Analysis between-group Principal Component Analyses (bgPCAs) were realised to obtain the areas seen in Figure 5b for the living groups. S. tchadensis and O. tugenensis data were then projected a posteriori in the bgPCA. The analyses were performed using the package ade4 v. 1.7e6 (Dray and Dufour, 2007) for R v. 3.6.3 (R Development Core Team, 2020).
The cross-section outlines presented in Figure 6 were obtained by extracting the cortical shell by manual delimitation of the endosteal and periosteal contours (SOM Fig. S4a). However, given some damage and the slight anteroposterior compression in the distal shaft (Fig. 3d), a projection a posteriori of two reconstructed outlines of the femoral shaft approximating its original contour in two ways was realized (SOM Fig. S4b, c). For comparison, the contours of femora representing H. sapiens, Pan, Gorilla, and Pongo were extracted together with the contours of an Australopithecus afarensis (“Lucy”) femur (SOM Fig. S5a) and of two fossil Great Apes: Hispanopithecus and Rudapithecus (the contour of this latter fossil was partially reconstructed to compensate for lateral damage and anteroposterior deformation; SOM Fig. S5d). (NB : A more exhaustive and precise description of the measurements and of the morphometric analyses can be found in the original article (not simplified)).
III. Results
1. Comparative analysis
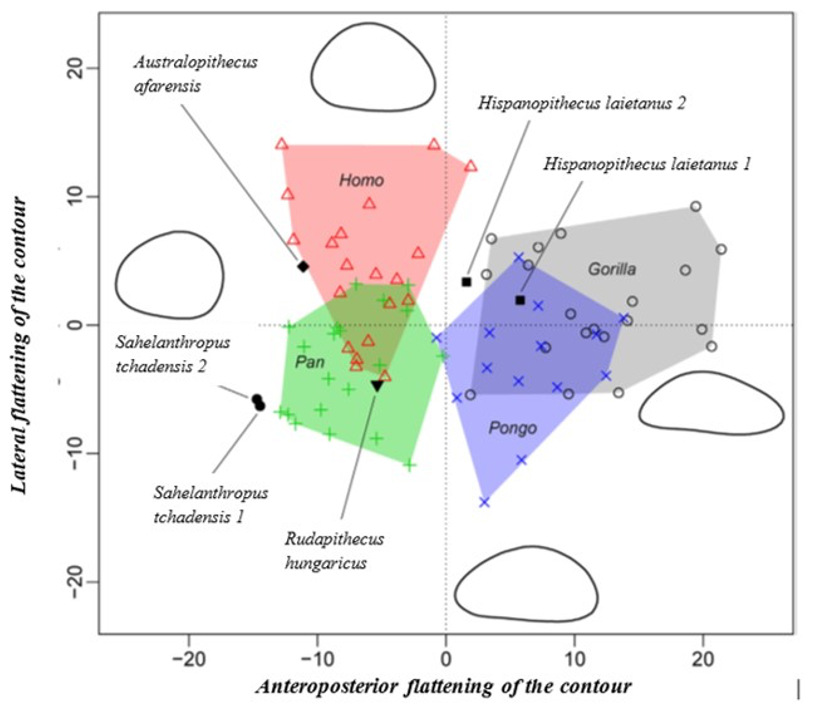
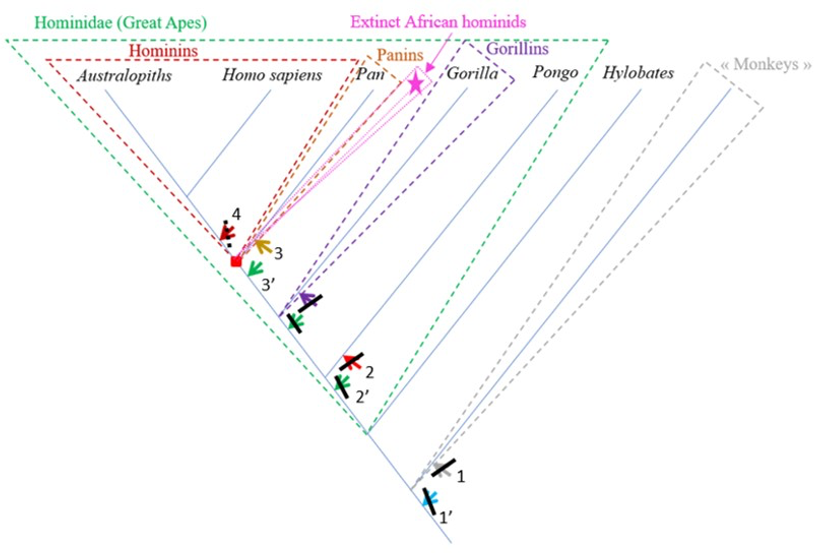
The characteristics of the femur of S. tchadensis were compared to those of the femora from the living hominid species H. sapiens, Pan, Gorilla and Pongo, but also to the fossil hominin species A. afarensis and O. tugenensis (2 better preserved femora) to establish its relationships with the different taxa and to determined where it can be positioned on a phylogenetic tree (see arrows on Fig. 10).
The results of the analysis presented in Table 2 indicate that S. tchadensis was not a monkey, but indeed a hominid (a Great Ape). Monkeys are Primates that have a tail whereas Great Apes lost it. These results exclude hypotheses 1 and 1’ on Figure 10.
The results of the morphological analysis of the shaft curvature (see Fig. 3c and Fig. 5b) separate, through the abscissa axis, Pongo from Homo, Gorilla and Pan, which have a more convex shape of the femur distally. They also separate Homo and Pongo from Pan and Gorilla (the African Great Apes), through the ordinate axis, that is likely to represent the flattening of a sinusoidal shape (Fig. 5b). So, this analysis reveals that Pongo is separate from other living hominids in terms of its femoral curvature and so in terms of locomotion.
The results of the analysis also show that S. tchadensis (see Fig. 5b) is closer to the common chimpanzee than to Homo, Gorilla and Pongo. So, these results (Fig. 9) indicate the hypothesis 3 on Figure 10 as more likely than the others, but they do not exclude the hypothesis 3’ on the same figure.
However, the same analysis tends to separate O. tugenensis from the living Great Apes, with a femoral shape intermediate between Pongo and Homo. This suggests that S. tchadensis and O. tugenensis may not have had the same type of locomotion.
The morphological analysis of the cross-sectional contours (Fig. 6b) shows that Hispanopithecus is separate from Modern Humans, far from Pan, and closer to Pongo and Gorilla. These results are consistent with its classification as a fossil Great Ape, so in a position near the arrows of the hypotheses 2 and 2’ in Figure 10.
For S. tchadensis, the comparative results of the cross-sectional femoral shape are closer to that of the common chimpanzee than to Homo, Gorilla and Pongo (Fig. 9). These results tend to indicate that the hypothesis 3 would be more likely, but they do not exclude hypothesis 3’on Figure 10. However, in S. tchadensis the cross sections of the shaft are larger than the average for Pan, possibly excluding hypothesis 3. But the neck-shaft angle estimated from the preserved morphology of S. tchadensis (Fig. 3b) ranges between 138°and 146° (SOM Fig. S1). If we use the conservative estimate of >135° for the Chadian fossil, it is likely to have been higher than the range seen in Homo, Pan, Gorilla and Orrorin, closer to the range of values for Pongo and Hispanopithecus (Köhler et al., 2002; Pina, 2016), thus favoring hypotheses 2 or 2’ on Figure 10.
Even if the diameter of the S. tchadensis’ cross sections are larger than the average for Pan (Table 2), the reconstructed biomechanical length of the femur is similar to the estimates for O. tugenensis (288 mm – 297 mm; Nakatsukasa et al., 2007; Puymerail, 2017; and original data). This suggests that this S. tchadensis individual likely weighted more than 47 kg, since this is the weight that was estimated for the largest of the two O. tugenensis individuals (Grabowski et al., 2018; see also Nakatsukasa et al., 2007).
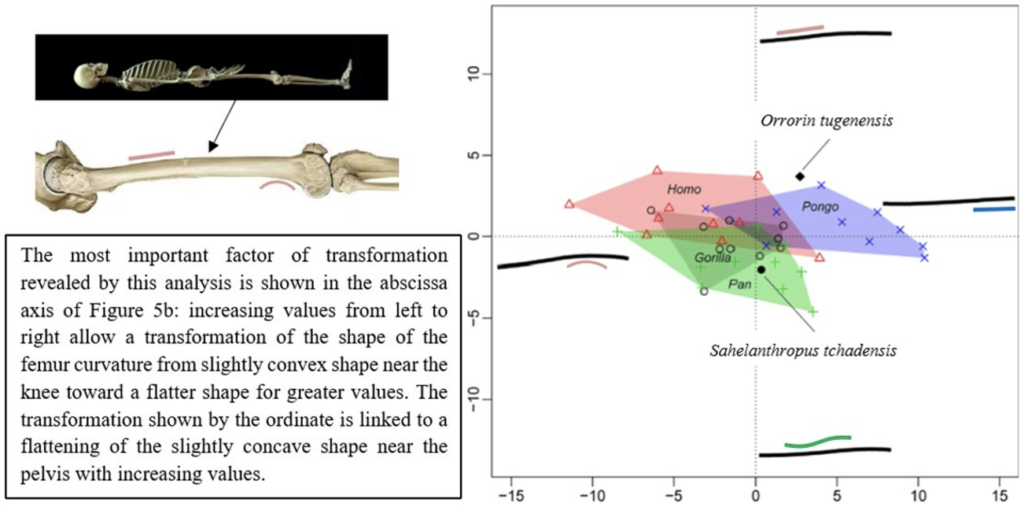
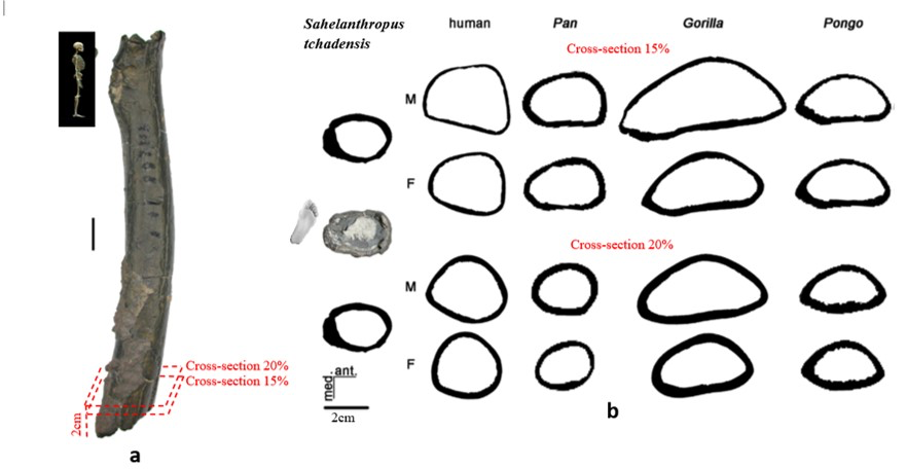
Figure 6.b Manual delimitation of the endosteal and periosteal contours of the cross-section of Sahelanthropus tchadensis femur at different level near the foot (cf. Fig. 3a) and of 2 virtual cross sections at 15% and 20% of the biomechanical length in a female (F) and a male (M) femurs representing Homo sapiens, Pan, Gorilla and Pongo.

2. Functional assessment
We often use bipedalism (upright walking) as a decisive factor to decide if a species belongs to the hominin group (e.g., Le Gros Clark, 1955), and so to determine whether Pliocene fossils identified as hominids (Great Apes) are from a hominin species or not (e.g., Haile-Selassie et al., 2004; White et al., 2009; Simpson, 2013; Pilbeam and Lieberman, 2017).
Even if all occasional bipeds may not be hominins, by definition hominins should be characterised by forms of habitual bipedal locomotion when on ground (not just occasional, like in Pan or even in Gorilla, that can use forms of bipedal displays to impress a rival, for example, or on short distances). So, showing that the morphology of the femur of S. tchadensis is consistent with habitual bipedalism would confirm its classification as a hominin.
To do this, two ways can be used:
– We can compare the morphology of S. tchadensis femur to Modern Humans, that are habitual bipeds, and to the other living Great Apes (Pan, Gorilla, Pongo), that are occasional bipeds, to see which one is most resembling. Here, the fossil specimen resembles common chimpanzees more than Modern Humans (Fig. 9). We also know that in hominins there is a neck-shaft angle that results in “touching” knees. This configuration implies a reduction in the width of the bone, so the bone becomes thinner from the part near the pelvis (proximal) toward the middle. The S. tchadensis femur does not show these characteristics, so it could be concluded that it was not a habitual biped. However, some fossil hominins do not show the changes present in Modern Humans either (Lovejoy and Heiple, 1972; Richmond and Jungers, 2008), even if they were almost certainly habitual bipeds, so the lack of these characteristics simply cannot be used to confirm if S. tchadensis represents or not an extinct hominin (on this unique basis, hypothesis 4 in Fig. 10 would be doubtful, but not formally excluded). Moreover, the upper and lower ends of the bone have unfortunately been destroyed and these are the parts where there is most information about the functional role (sensu Bock and von Wahlert, 1965) of the femur (e.g., Lovejoy, 1988; Richmond and Jungers, 2008; Ruff and Higgins, 2013; Marchi et al., 2017; Cazenave et al., 2019; Pina et al., 2019; Sukhdeo et al., 2020). Accordingly, a lot of pertinent information is missing to conclude this way.
– Or, we can compare the morphology of S. tchadensis to the most complete femur of O. tugenensis (Senut et al., 2001; Pickford et al., 2002). Indeed, most scientists that studied its femurs agreed that O. tugenensis probably displayed a form of habitual bipedalism similar to the one found in Australopithecus (Pickford et al., 2002; Galik et al., 2004; Nakatsukasa et al., 2007; Richmond and Jungers, 2008, 2012; Kuperavage et al., 2018), even if Almecija et al. (2013) found some differences with respect to the Australopiths, and Ohman et al. (2005) questioned the interpretation of its internal morphology. Moreover, if we consider more particularly the morphology of the proximal epiphysis, Bleuze (2012) also concluded, after a comparison of the geometry of its cross-sections, that O. tugenensis shows bipedalism similar to the Australopith locomotion mode. Thus, if we follow the logic of our preceding argument, if O. tugenensis displayed a bipedalism similar to the Australopith pattern, it was probably a habitual biped and so, if S. tchadensis femur had similar characteristics, then we could use it to support the hypothesis that it was probably a habitual biped too.
Even if the proximal epiphysis is missing in S. tchadensis, this study was still able to compare the femoral morphologies of S. tchadensis and O. tugenensis (Fig. 5 to 9).
The analysis of the anteroposterior curvature and the neck-shaft angle in this study does not show enough similarities between the two fossil species to conclude on S. tchadensis bipedalism being similar to O. tugenensis.
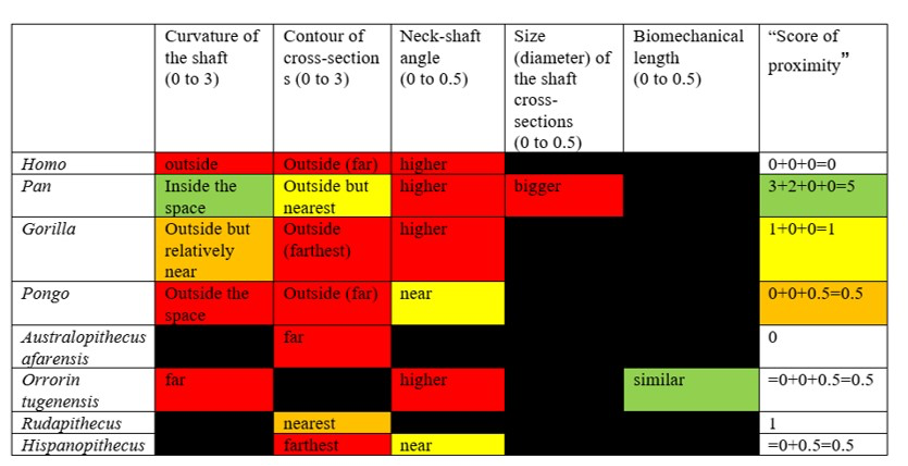
Moreover, when considering the bony characteristics that could have a functional significance in terms of bipedalism, the picture that can be drawn is very difficult to interpret because the characteristics present in O. tugenensis or in any living or fossil apes have not been found in S. tchadensis, whereas some characteristics specific to the Chadian specimen have not been found in other species. Indeed, Table 3 highlights the fact that the different characteristics found on femora that could have a functional role in bipedalism are not consistently found in different groups. These different features seem to be different or combined differently in each group, so they do not allow any conclusion on S. tchadensis habitual bipedalism and each feature cannot be used to conclude that S. tchadensis was a habitual biped, so, an early hominin. Nevertheless, we can confidently state that S. tchadensis locomotion pattern differed from that of O. tugenensis.
IV. Discussion
Guy et al. (2005) explained that more research and more information are needed to define the relationship between S. tchadensis and known Miocene and Pliocene hominids/hominins and to confidently position it on the phylogenetic tree of Primates (see Fig. 10). Thus, to prove that S. tchadensis was indeed a habitual biped (and so a hominin) as suggested following the first research on the cranium, Richmond and Jungers (2008: 1662) wrote that “postcranial fossils (i.e. fossils that are parts of the body other than the skull) are needed to confirm this conclusion”.
So, in the following paragraphs it will be presented, in descending order of confidence, what can be said at this stage from the results of this study about the functional morphology of S. tchadensis femur and, consequently, what this implies in terms of relationships between this species and other living and fossil species/groups. Moreover, since the information provided by this study is mostly limited to the shaft, it is important to keep in mind some carefulness about the conclusions reached here.
The most reliable and parsimonious working hypothesis is that the studied femur indeed belongs to S. tchadensis and so that S. tchadensis was a hominid sensu lato (which places it in the “green box” of Figure 10, thus excluding hypotheses 1 and 1’).
There are important differences between S. tchadensis and O. tugenensis femora indicating that they belong to different species, and maybe even to different groups. Finally, if the studied femur indeed represents S. tchadensis, the results of this study on its preserved morphology imply that it may not have been a habitual biped so, by extension, that it may not have been a hominin (which would exclude him of the “red box” in Fig. 10, thus excluding hypothesis 4).
But it is important to say that the observations on the femur are only partial, since they are limited to its outer morphology and to what can be currently found in the literature, plus limited by a brief access to the original fossil. So, we realize that if those with curatorial responsibilities for the original specimen will conduct a more detailed and thorough comparative study, including assessments of its cross-sectional geometry and internal structure, the conclusions may be different.
On the other hand, if the studied femur indeed belongs to S. tchadensis, what are the implications of the results of this study on the evolutionary relationships of S. tchadensis? Will our understanding of hominid and hominin evolution in Africa between 7 and 6 Myr change?
There is a lot of differences between the morphology of chimpanzees/bonobos and that of Modern Humans, but the difference between the ancient Miocene ancestors of Modern Humans and chimpanzees were likely to be less obvious. Among the characteristics that distinguish Modern Humans from chimpanzees, the ones related to bipedalism seem to go far back in time (Almécija et al., 2013; Böhme et al., 2019). Other characteristics like smaller jaws and molars seem to have evolved more recently in the human lineage and so cannot be used to confidently differentiate a “primitive” hominin (hypothesis 4 on Fig. 10) and a “primitive” panin (hypothesis 3 on Fig. 10).
But, with this caution, how could we differentiate a “primitive” hominin and a “primitive” panin?
The common hypothesis is that “primitive” panins should have had a face with a long jaw (strong prognathism) with rather small molars, big honed canines with a significant difference between males and females (Pilbeam and Lieberman, 2017), and a body adapted to a quadrupedal-like locomotion in the trees. On the other hand, a “primitive” hominin should have had cranial, skeletal and other locomotory adaptations that allowed being able to stand by and walk upright for a long time. These characteristics would have been combined with relatively large molars and “small” canines. These inferences are working hypotheses that will be revised when the evidence is discovered (Guy et al., 2005).
Indeed, the presence of one or some characteristics that can distinguish the first hominins from the first panins is likely not to be sufficient to identify a fossil as a hominin or panin, since there is evidence that Primates, like many other mammalian groups, are affected by homoplasy (aka false homology; Diogo and Wood, 2011). This means that some characteristics that appear similar (or have a similar function) can result from separate evolution and so do not imply that the species that possess them have a common ancestor. For example, after a climatic cooling in the environment, snow may become a regular occurrence and a white color may be advantageous. So, some beetle, rabbit and mouse individuals that have separate random mutations resulting in a white color may be preferentially selected and become common in the different populations, but this similarity certainly does not mean that the common ancestor of beetles, rabbits and mice was white. Thus, the possibility of homoplasies means that it is not impossible, indeed it may even be probable, that some of what many have come to regard as key morphological adaptations at the base of the hominin lineage may have evolved separately more than once. If that is the case, then one or two features can’t be used to define a species, but particular sets of features should rather be used to separate a group from another.
It is possible that S. tchadensis is a “primitive” hominin with some reduction of the canine and loss of the honing complex (hypothesis 4 on Fig. 10), but without the femoral adaptations to terrestrial bipedalism that are seen in A. afarensis and O. tugenensis (Pickford et al., 2002; Galik et al., 2004; Richmond and Jungers, 2008; Almécija et al., 2013). It has been suggested that the early Pliocene Ardipithecus ramidus (Lovejoy et al., 2009; White et al., 2009; Simpson et al., 2019) was a biped, but it is difficult to see how the partially opposite position of its big toe is compatible with an obligate terrestrial bipedality. Based on this study, S. tchadensis femur lacks any feature consistent with habitual bipedalism; instead, its general characteristics suggests a derived Pan-like morphology. Thus, either we consider that S. tchadensis was not a “primitive” hominin or, if S. tchadensis is included among the current hominins, then bipedalism can no longer be seen as a requirement to belong to hominins.
But being a “primitive” hominin or a “primitive” panin, or their most recent common ancestor (red square on Fig. 10), may not be the only options for S. tchadensis. Given what we have learned about the evolutionary history of the hominids, it is likely, and indeed probable, that during the late Miocene and the early Pliocene, there was a modest adaptive radiation of African hominids that includes taxa that are neither hominins nor panins as defined previously (Wood and Harrison, 2011). Any such extinct groups are likely to include taxa with novel morphologies, or with novel combinations of characters we also see in hominins or panins. Given the mix of inferred primitive and inferred derived features in S. tchadensis, we suggest this Miocene species could belong to a group of extinct hominids that has no living representative (see pink dashed group and star on Fig. 10).
V. Conclusion
The lack of proof allows only to say that S. tchadensis was a hominid but we can’t be sure that it was a habitual biped, so it seems even less likely than it was before that S. tchadensis could be a “primitive” hominin (Mongle et al., 2019). However, this does not make the discovery of this species less important (Brunet et al., 2002). There is lot of proof that shows that, for at least the last four million years, there is a diversity of lineages within the hominin group (Haile-Selassie et al., 2016; Wood and Boyle, 2016). So, it would mean that there are high chances that the fossils that have been found may not be direct ancestors of Modern Humans but rather would be “cousins”, but it is currently difficult to sort direct ancestors from non-direct relatives (since we lack information on these extinct branches that have no living descent). So, there is no logical reason to think that the same problems and limitations do not also apply to the late Miocene hominids like S. tchadensis. It will not be easy, especially since the fossils are so few, to work out which late Miocene species are hominins, which are panins, and which are neither. As one of us had previously suggested, “exactly where in Africa, and under what circumstances, the ape-human demarcation began, and when, how and why the ape-human boundary became irrevocably established, are important research challenges that are still unresolved” (Wood, 2017: 103). But if we treat the hominin status of S. tchadensis, or any other enigmatic species, as a given and not a hypothesis, we run the risk of adding further confusion to a picture that is already “complicated and less easy to resolve” (Guy et al., 2005: 18839).
References :
(see references original article)
Ont participé au travail d’écriture de cet article, en collaboration avec Roberto Macchiarelli, chercheur en paléobiologie et paléoanthropologie (par ordre alphabétique): Léon ALARD; ALLART Lubin; BEAUFORT Callixte; BELLOT Simon; CHAUDERLIER Gaylor; CHENU Rémi; CHERPIN Aude; COQUENET Théo; COUPEZ Jules; DEHUT Raphaelle; DEPIL Nathan; DESSITER Axel; DUPRE-ROSIN Yael; FOUQUART Audrey; GAUDION Ophélie; GAUME Gabrièle; LEVEBVRE Eloïse; LEFEVRE Paul Emmanuel; PARENT Léa; PRINCE Juliette; RENAUD Margot; RENAUD Victoire; REVUE Marius; ROUSSELLE Léa; RUTKOWSKI Margot; SEGUIN Lucie; STEVENIN Romane.
Comment citer cet article : Roberto Macchiarelli et la Terminale Euro (TEURO) du lycée Joliot-Curie (Hirson (FR)), Nature and relationships of Sahelanthropus tchadensis, Journal DECODER, Date (2022-10-21).
